Life Science Washington’s Member Spotlight series highlights the innovations, impact, and future vision of our member companies in the life sciences industry. Through curated questions, we explore their origins, breakthrough technologies, local presence, and industry leadership. This initiative showcases their contributions and strengthens connections within the life sciences community. If your organization is a member and would like to be highlighted in a future spotlight, please contact Kaitlyn Campitiello, Director of Marketing and Communications.
Tell us your origin story. How did Lundbeck come to be, and what inspired its creation?

Lundbeck was founded in 1915 by Hans Lundbeck in Copenhagen, Denmark, originally as a small trading company. By the mid-20th century, Lundbeck shifted its focus to healthcare and established itself in the treatment of central nervous system (CNS) disorders. With pioneering developments in psychiatric and neurological treatments, Lundbeck became one of the few pharmaceutical companies solely dedicated to brain health.
As a focused innovator, we strive for our research and development programs to tackle some of the most complex neurological challenges. We develop transformative medicines targeting people for whom there are few or no treatments available, expanding into neuro-specialty and neuro-rare from our strong legacy within psychiatry and neurology.
Which therapeutic areas is Lundbeck most focused on advancing right now, and what drives that focus?
Lundbeck is focused on advancing treatments for neuro-specialty and neuro-rare conditions. These areas represent high unmet medical needs. With over 70 years of expertise in brain health and over 30 treatments launched globally, Lundbeck is committed to developing transformative medicines that improve the lives of patients, their families, and society.
Over the last five years, the company has significantly increased its share of neuro-rare and neuro-specialty programs from 40% to nearly 90% of its pipeline, demonstrating our commitment to focused innovation. This strategic focus allows us to target defined patient populations and deliver meaningful solutions that address critical gaps in care.
Lundbeck is also conducting over 10 clinical trials across 20+ countries, investigating if the solutions have the tailored potential to meet the needs of patients and society.
How does the Lundbeck Bothell site support Lundbeck’s global goals? Is there a continued focus on Alder Biopharma assets, or has the scope evolved?

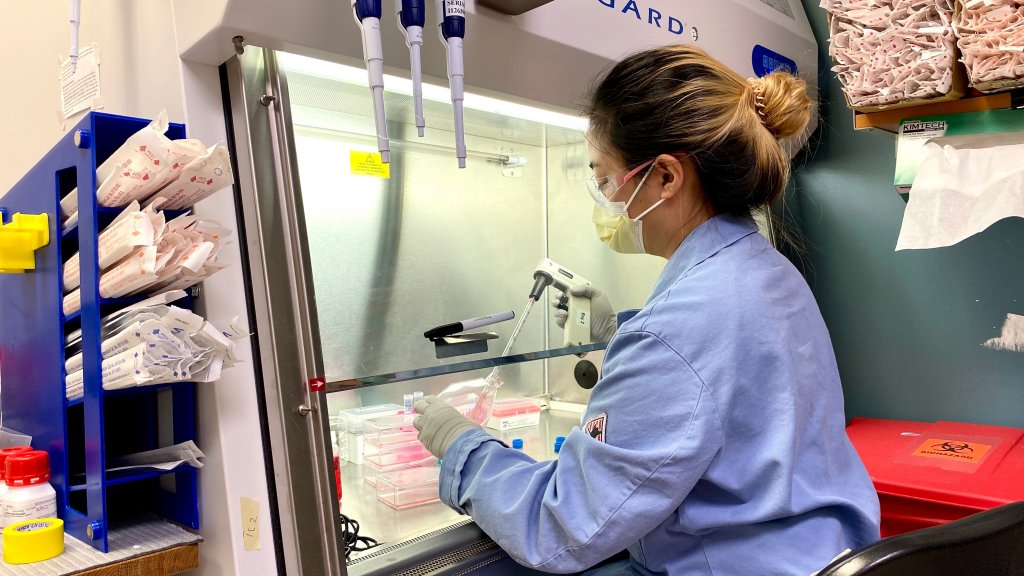
The Lundbeck Bothell site is home to our Center of Excellence for Biologics Process Development, where we develop and refine manufacturing and analytical processes for our biologics programs, both for early-phase molecules just transitioning out of Research and for later-phase molecules requiring more in-depth or subtle analysis and development. We also have team members in Bothell who support other aspects of our global biologics programs, such as Quality, Process Science, and Validation. The Bothell site is critical to helping our clinical and commercial biologics portfolio to advance and continue serving more patients. We support all Lundbeck biological assets.
What’s a recent scientific challenge your team solved that had a direct impact on moving a therapy forward?
Recently, our Formulation Development staff were able to carry out a number of studies very quickly and propose a new formulation for a molecule in an early clinical phase that provided substantially better stability and thus much better shelf life, which has the potential to improve patient access to this therapy in the future. This is a great example of the work that we do to make our manufacturing processes better and to tailor our development work to fit each molecule that we work on.

How does the work being done at the Bothell site, from process development to manufacturing, translate into real-world therapies for patients?

We have recently implemented a pilot lab at the Bothell site. This enables us to produce quantities of protein at a scale and quality suitable for toxicological studies. That ability in turn allows us to initiate toxicological studies up to nine months sooner than if we transferred the process for tox manufacturing to a contract manufacturing organization (CMO). And that earlier initiation of tox studies enables us to shorten all our project timelines, allowing us to deliver our new and innovative biologics therapies to patients faster in the future.
Where do you see Lundbeck in the next 5-10 years? Can you share some of your most exciting upcoming projects or innovations?
We are now more than a year into the Focused Innovator journey and we are seeing significant progress. Our strategic initiatives focused on growth, innovation, and funding are paying off, establishing a strong foundation for long-term growth.
Looking ahead to 2027-29, our goal is to continue to grow our migraine and neuro-rare franchise, looking at additional growth opportunities in these areas. We will also explore R&D partnerships, in addition to commercial partnerships that would allow our treatments to reach more patients in more geographies. From 2030 onwards, we anticipate seeing our organic pipeline bearing fruit and demonstrating the true potential of our best-in-class neuroscience research discovery platform, alongside ongoing business development efforts.
Learn more about Lundbeck Biopharmaceutical and stay updated by following their social channels:
- Lundbeck’s Website: https://www.lundbeck.com/us
- Lundbeck’s Career Site: https://www.lundbeck.com/us/careers
- Lundbeck‘s Social Channels:
- LinkedIn: https://www.linkedin.com/company/lundbeck
- X: https://x.com/LundbeckUS
- Instagram: https://www.instagram.com/h_lundbeck/
If your organization is a member of Life Science Washington and would like to be highlighted in a future spotlight, please contact Kaitlyn Campitiello, Director of Marketing and Communications.